- | 1:27 pm
This startup uses an ancient microbe to turn CO2 into ingredients for food

Inside bioreactors in a Vienna-based lab, the startup Arkeon Biotechnologies is reimagining farming: Using a single-step process of fermentation, it’s turning captured CO2 into ingredients for food. Unlike other fermentation processes—such as brewing beer—it doesn’t start with sugars from plants. Instead, the company uses a microorganism with the unique ability to directly transform CO2 into the building blocks for carbon-negative protein.
“The unique feature of the microorganism we’re using is that it’s producing all of the amino acids that we need in human nutrition,” says Gregor Tegl, the CEO of Arkeon, which just raised a seed round of $7 million from investors, including Synthesis Capital and ReGen Ventures. “And it’s also spitting them out of the cell just naturally, which is an insane thing to do.”
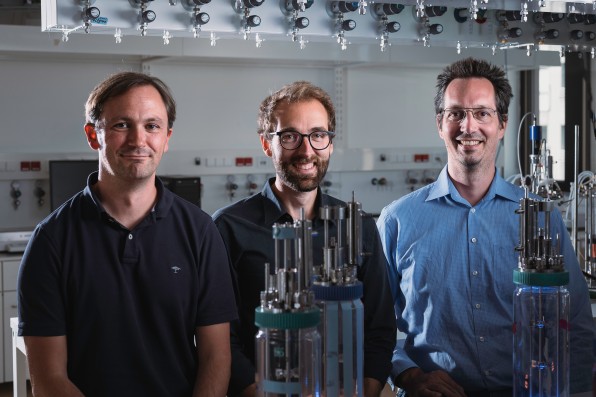
[Photo: courtesy Arkeon]
The resulting ingredients could be used in alternative protein products, such as plant-based milk or meat. Right now, if a food company is making a plant-based burger with something like pea protein, it involves a complicated process of purefying the protein to remove unwanted flavor, and often also involves adding additional ingredients to help mask the taste. By creating amino acids from the bottom up, so they’re already pure, Arkeon eliminates processing and additives. The amino acids can then be combined to create tailored ingredients that mimic the mouthfeel and flavor of meat, which the company thinks can help expand the number of alt-protein foods on the market. The ingredients can also be used directly in protein drinks and infant formula.
That’s a huge contrast with protein from animal agriculture, one of the most climate-intensive industries that exists. “Protein production using animals is the most inefficient and at the same time the most cruel process you can imagine,” says Tegl, who is vegan. “And that’s exactly our motivation, why we started Arkeon—because we saw the potential of technology to actually make that obsolete.”
In a few weeks, the company will expand to a pilot facility to begin producing its ingredients at a larger scale. It’s partnering with breweries to use CO2 captured in the brewing process; breweries can use some of the CO2 themselves for carbonating drinks, but typically only use a fraction. The company can also use CO2 from bioethanol production. In both cases, the CO2 is pure, so it doesn’t have to be refined further before it can be used in food. The startup is also working on getting regulatory approval, which it says will be easier in Europe than it would be for some similar companies because its microbes aren’t genetically engineered. Eventually, it will begin large-scale production in larger tanks. “It will actually look very similar to breweries,” Tegl says.