- | 8:00 am
This startup is growing mini-livers to keep patients alive
Dimension Bio wants to grow a simplified liver from cells to sustain patients long enough for a damaged liver to recover—or until a transplant arrives.
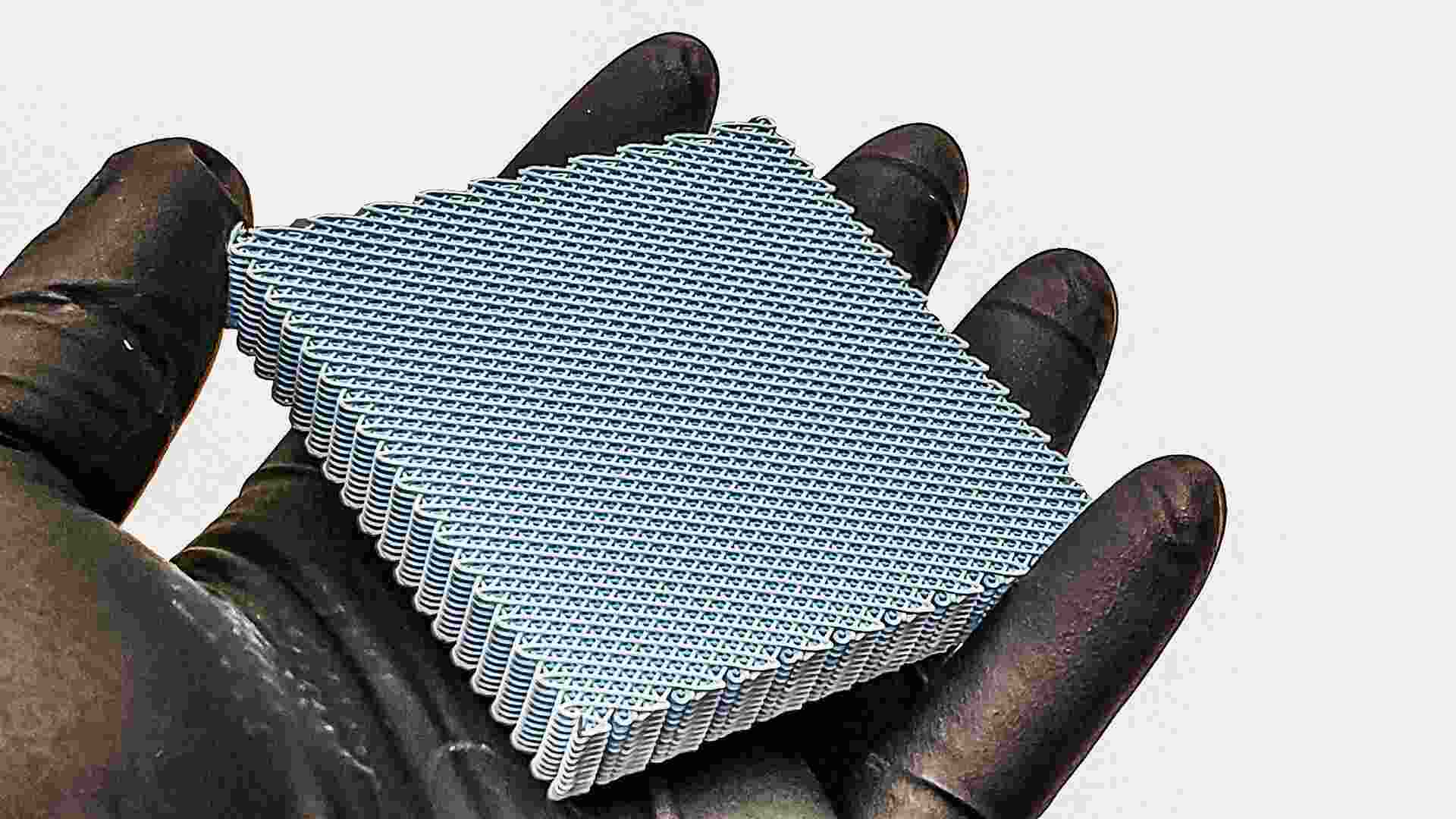
Caralynn Nowinski Collens, Ramille Shah, and Adam Jakus spent years developing an innovative technology to regenerate injured bone. The results, they thought, were . . . okay. The company they founded, Dimension Bio, received clearance from the Food and Drug Administration for its approach: providing a 3D-printed lattice or “scaffold” for new bone to grow in. However, it didn’t form new bone fast enough to compete with established treatment methods, such as transplanting a patient’s own bone tissue.
But Collens, Dimension’s CEO, sees the experience as a net positive, validating the company’s technology and processes with the FDA. That could help the Chicago-based startup work toward a more-ambitious goal in about three years: building a human liver using its scaffold and donated cells. It would actually be a miniature, simplified version of the organ, meant to function well enough to keep someone alive. That could provide breathing room for an injured or diseased liver to heal, or buy time for the patient to receive a transplant.
According to the Centers for Disease Control and Prevention, 52,222 people died of liver disease and cirrhosis in 2023. The death rate from cirrhosis increased 26.4% from 2000 to 2019, per the National Institutes of Health. (Cirrhosis is most often the result result of fat buildup, viral hepatitis B and C, or long-term alcohol abuse, though there are other causes.)
“The mortality rates are very high when a patient can’t get a transplant. And so that’s where we’re looking to be able to provide [help],” Collens says.

Reprinting Older Science
Building a scaffold for cells to grow in is not novel, Collens concedes, nor is the material the company uses: poly lactic-co-glycolic acid. PLGA is found throughout everyday medicine, including in dissolving sutures, dermal fillers, and tiny capsules to deliver drugs to the body.
Dimension Bio’s innovation is in how it utilizes 3D printing to build the PLGA scaffold, part of an overall system it’s dubbed BioNidum. “[When] we put that in the body, what happens is it gets new blood vessels very quickly, and that’s unusual,” says Collens, noting that typically the immune system walls off the foreign body and prevents blood vessels from growing easily.
Collens attributes the success to a scaffold structure that provides pores of different sizes, designed to help cells move into the scaffold easily and not provoke a strong immune response. The larger pores allow the blood vessels to grow into the new tissue.
The company was originally called Dimension Inx, a bit of wordplay. “We make biomaterial inks,” Collens says.
The technology grew out of Northwestern University’s TEAM lab, short for tissue engineering and additive manufacturing (a fancy name for 3D printing), founded by Shah in 2010. At the time, Collens was running an advanced manufacturing innovation center in Chicago, part of a national network of institutes funded by the federal government and companies including Microsoft and Lockheed Martin. In 2015, one of the board members sent Collens an email, “and he said, ‘I just saw this rock star young faculty member present, and I think you should meet her,’” she recalls.
Shah, a materials science professor at the time, and then-graduate student Jakus founded Dimension Inx in 2016, and Collens joined as a cofounder and CEO 2019. Jakus left the company in 2023. Shah serves as chief science officer. She, Collens, and three other women account for all voting members on Dimension’s board of directors. The overall staff is about “70% diverse,” Collens says. “I’ll say it’s intentional only in the fact that we have a strong belief, and it’s one of the values of the company, that diversity leads to better outcomes and better innovation.”
The company raised $20.52 million through seed rounds in 2020 and 2021, and a series A in 2023. Lead investors include KdT Ventures and Prime Movers Lab. Another major investor is Revolution’s Rise of the Rest Seed Fund, which focuses on startups outside top investment regions and doesn’t typically fund biotech. The company is planning what it calls a series A2 funding round in 2026, with the goal of raising up to $50 million.
Boning Up on the Technology
CMFlex—the company’s earlier bone repair product—is considered a “medical device,” requiring a less-stringent FDA review than medications or Dimension’s upcoming mini-liver. That’s because CMFlex is just the scaffold for the patient’s own cells to grow into, rather than to introduce new cells. Bone provided the “lowest hanging fruit,” for Dimension to prove out its technology, Collens says, because it’s a naturally regenerative tissue. “We were putting this matrix or scaffold inside to serve as a guide or an instigator to get new bone.”
The FDA didn’t require human trials for this medical device. Although it had success in animal studies, Dimension chose to do a pilot program in patients before making the product available. “We have lots of examples of being able to create bone in patients and in animals,” Collens says, adding, however, “We didn’t do it fast enough for the structure that’s needed—and the structure meaning the hardness to withstand the forces that bone allows you to withstand.” Dimension eventually decided working with tissue would be more impactful, and decided not to go to market with CMFlex.
Moving to soft tissue and then organs was part of the original pitch deck to investors. The company is investigating restoring function in ovaries, for instance. It also succeeded in growing insulin-producing cells seeded in its scaffold in diabetic mice, which could pave the way for treating diabetes in humans. But that area was already a crowded market, Collens says.
“We ended up focusing on liver failure for a variety of reasons, but probably one of the biggest reasons is it’s a huge problem with no good alternative, except for liver transplant,” she says.
Dimension’s plan is to grow a small, simplified liver under the skin as a temporary fix until either the full liver can recover or the patient can get a transplant. “I think that’s a good way to go,” says James Anderson, a retired professor of pathology, macromolecular science, and biomedical engineering who taught at Case Western Reserve University for more than 40 years. Anderson, who is not associated with Dimension Bio, reviewed its research and was impressed with the methods and results. The liver, he says, is not only a worthy target for regenerative medicine; it’s also a conducive one. “They picked an organ that can reproduce itself,” he says.
But even a mini-organ is much more complicated than bone. “It’s a fundamentally different type of product, when we’re talking about putting cells on a scaffold,” Collens says.
In mice whose livers were deliberately damaged, the company reports that it increased the survival rate by more than 70% after seeding with liver cells from mice and humans. That required hundreds of millions of cells. But according to Collens, building the miniature human liver could require seeding the scaffold with 5 billion to 20 billion cells.
For humans, Dimension will use stem cells to produce those billions of liver cells. But first come tests in rats and pigs. The company’s timetable is aggressive. It aims to start clinical trials in humans in 2028.
The next question might be: Why not grow an entire liver replacement? That seems to be, at best, a very distant goal. Anderson is not sure it’s possible, given the complex structure of the full organ. Collens says Dimension Bio is not working on that lofty goal, for now. But she doesn’t rule it out.
“I think we’re at a really interesting inflection point . . . of this convergence of engineering and biology, where we can actually engineer biological systems that support function that we couldn’t do before.”